On Wednesday, we received our Stoichiomety quiz grades back and I got a 100%. I was so relieved. My hard studying the night before paid off. Now, I am preparing for the unit exam. Below are a couple helpful links with practice quizzes and tests that will help me learn more about the subject. Hope you find these helpful!
Somerset Academy Practice Test
Glencoe Practice Quiz
Sunday, December 13, 2015
Day 1 and 2 of Copper (II) Chloride and Iron Lab
On Thursday and Friday, my lab partner and I performed an experiment where we produced copper from an iron nail and copper(II) chloride. On day one, after we passed our pre-lab quiz, measured about 4 grams of copper(II) chloride, and placed it in a baby food jar. It was a vibrant blue. Next, we added 50 mL of distilled water to the jar and stirred it until it dissolved. We then placed an iron nail that had been polished with steel wool in the jar to sit overnight. Within in minutes it looked like this...

 You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
In order to separate the copper from the rest of the solution, we pulled out the rest of the iron nail, washing off the remaining copper on the nail with distilled water back into the baby food jar. Then, we siphoned off the solution of iron (II) chloride into the sink, careful not to lose any of the copper the reaction produced. We then added hydrochloric acid to the baby food jar to wash the copper and also siphoned it off into the sink. Lastly, we washed the copper again with distilled water and siphoned off the water. Finally, we left the copper in the baby food jar and the nail on a paper towel to dry. We will finish measurements this week.

 You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
In order to separate the copper from the rest of the solution, we pulled out the rest of the iron nail, washing off the remaining copper on the nail with distilled water back into the baby food jar. Then, we siphoned off the solution of iron (II) chloride into the sink, careful not to lose any of the copper the reaction produced. We then added hydrochloric acid to the baby food jar to wash the copper and also siphoned it off into the sink. Lastly, we washed the copper again with distilled water and siphoned off the water. Finally, we left the copper in the baby food jar and the nail on a paper towel to dry. We will finish measurements this week.
Wednesday, December 9, 2015
Limiting Reagent and Excess Reagant
On Monday, we learned how to find the limiting reagent and excess reagent in a chemical reaction. There are two approaches. I prefer to use approach two. Below are the steps:
When solving for the limiting reagent, you must convert both of the reactants in a chemical equation to one of the products, using moles. Whichever conversion results in a smaller product, that is the limiting reagent. It is the limiting reagent because once that reactant runs out, the reaction will end and the other reactant will be left in excess. After determining the limiting reagent, you know the other reactant is the excess reagent. You can figure out how much was left in excess by converting the amount of the product that resulted from the previous limiting reagent conversion back to the amount of the excess reagent. Once you discover how much reacted, you can then subtract that number from the original amount and you will know how much is left after the reaction ends.
This picture show the reactants before and after the reaction, distinguishing which is the limiting reagent and what is leftover and alone as the excess reagent.
 |
| http://www.mhhe.com/physsci/chemistry/chang7/ssg/chap03_9sg.html |
Below is a link that explains limiting and excess reagents and another link with practice problems.
Tuesday, December 8, 2015
New Unit
On Friday, we began our new unit: Stoichiometry. Stoichiometry involves the relationship between the reactants and products in chemical equations. In the lecture, we balanced equations and followed a flow chart (the one below) to find the grams and moles of its different products and reactants.
Here is a video that explains Stoichiometry and its significance and a link to where you can practice simple Stoichiometry problems.
Video
Practice
Here is a video that explains Stoichiometry and its significance and a link to where you can practice simple Stoichiometry problems.
Video
Practice
Monday, November 30, 2015
Classifications
There are three umbrellas of chemical reactions:
- Formation of a solid
- Formation of water
- Transfer of elections
In the formation of a solid, double replacement reactions take place. On the reactants side, both reactants must be aqueous. On the product side, one product is insoluble and one product is soluble.
In the formation of water, a strong acid and a strong base must react in order to form water and a salt. If the acid and base are not strong, they will not split. Double replacement reactions also take place in this type of reaction.
If the reaction does not fit into either of these categories, it is a redox reaction. There are four types of redox reactions: single replacement, synthesis, decomposition, and combustion.
Here is a link to practice recognizing reactions: Classification Quiz
Wednesday, November 25, 2015
Transfer of Electrons: Redox notes
In class yesterday, we learned about redox reactions and the different types. In redox reactions, electrons are transferred from the metal to the nonmetal. If a species loses electrons, it is said to be oxidized, and it is the reducing agent. On the other hand, if a species gains electrons,it is said to be reduced, and it is the oxidizing agent. A way to remember this is:
In redox-single replacement reactions, the metals change particles and the driving force is the transfer of electrons. The reaction is based on a reactivity series. Something to remember is "like attacks like." So, a metal attacks a metal, and a nonmetal attacks nonmetals. Below is an example:
Next, we practiced synthesis reactions. Synthesis reactions occur when two or more reactants combine to form one product. The general formula is A + B = AB. Decomposition reactions are the opposite, so one reactant produces two or more products. The general formula for decomposition reactions is AB = A + B.
Here are a couple videos that further explain redox reactions:
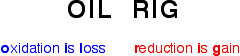 |
| http://www.chemguide.co.uk/inorganic/redox/definitions.html |
 |
| http://makahiki.kcc.hawaii.edu/chem/single_displ_rxn.html |
Lastly, we reviewed combustion reactions as our last redox reaction. In a combustion reaction, the reactants always include water, and produce carbon dioxide and water. Below is an example:
| http://www.angelfire.com/un/sch3u1/combustion.html |
Monday, November 23, 2015
Reactions that form water notes
Today in chemistry class, we took notes over reactions that form water. These reactions include an acid and a base, and the driving force is the production of water. A salt is also produced. For example:
| http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/acidbase.html |
Acids can be strong or weak and bases can be strong or weak.
Strong acids:
- produce H+
- protonate completely
- are strongest when oxygens outnumber the hydrogens by 2 or more
- HCl, HBr, HI
Strong bases:
- contain an -OH anion
- completely disassociate
- All group 1 and 2 metals cations plus an -OH anion are strongest
Weak acids and weak bases:
- do not protonate completely
- are not on the memorized list
Here is a link that further explains strong/weak acids and bases: ChemTeam
Here is a link to practice determining is an acid is strong or weak: about chemistry
Below is also a molecular, complete, and net force equation using strong acids and bases that we solved in class:
Wednesday, November 18, 2015
Driving Forces in Chemistry
We learned a somewhat confusing lesson in chemistry class today. Mrs. Frankenberg discussed driving forces in chemical reactions. Two components will react if there is at least one driving force present. The driving forces of chemical reactions are:
-Formation of a solid
-Formation of water
-Formation a gas
-Transfer of electrons
There is a link to a video below that explains these and indications of chemical reactions
-Formation of a solid
-Formation of water
-Formation a gas
-Transfer of electrons
There is a link to a video below that explains these and indications of chemical reactions
However, we mainly focused on the formation of a solid today, which is also known as precipitate. With these, double replacement precipitation reactions take place. In a double replacement reaction, 2 compounds replace 2 compounds by having the positive ions (cations) switch with each other. The format of this reaction looks like this:
 |
| http://socratic.org/questions/is-this-reaction-a-double-replacement-reaction-fe-c5h5-2-bf4-nab-c6f5-4-fe-c5h5- |
In addition, for this type of reaction to take place, the compounds that are the reactants must be ionic and aqueous and one of the products must be a solid. We must memorize the solubility rules to check if a chemical equation has a driving force.
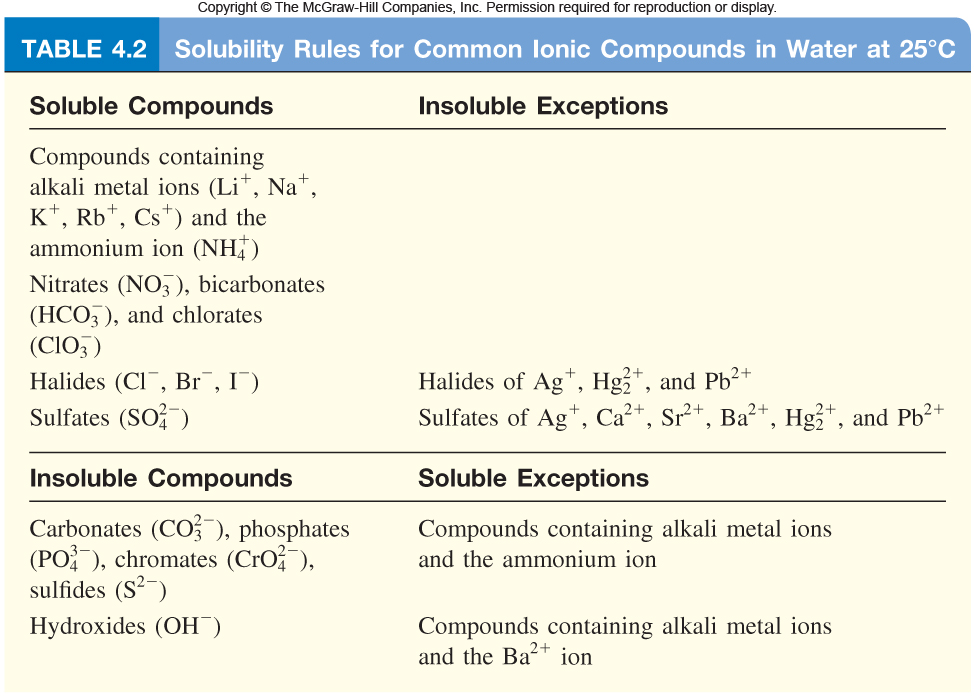 |
| http://highered.mheducation.com/olcweb/cgi/pluginpop.cgi?it=jpg::::::/sites/dl/free/0023654666/650262/Solubility_Rules_4_02.jpg::Solubility%20rules |
Tuesday, November 17, 2015
Start to a new unit
Today in chemistry class, we started our new unit: Chemical Reactions. We learned about several new and old concepts . I didn't find the lesson too difficult. However, I do need to review a few old ideas. First, we talked about clues of chemical reactions, and how they are different from physical changes. Below is a picture of some chemical reaction changes.
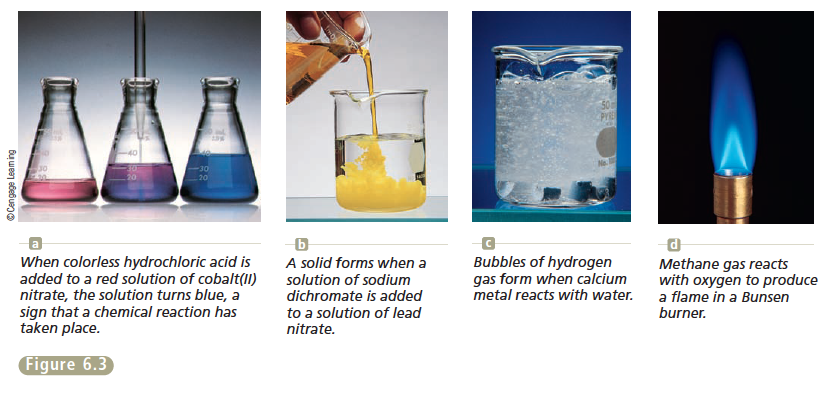 |
| http://chemistrysaanguyen.weebly.com/61-evidence-for-a-chemical-reaction.html |
Next, we discussed chemical equations and reviewed how to balance them. We learned that we can only change coefficients and not subscripts. In the picture below, you can see that the equation is balanced. On the left (reactants) there are 4 hydrogens because the hydrogen coefficient of 2 multiplies with the 2 subscript below the hydrogen. There are also 2 oxygens on the left. On the right (products there are 4 hydrogens and 2 oxygens to balance the equation.
 |
| http://www.mikeblaber.org/oldwine/chm1045/notes/Stoich/Equation/Stoich01.htm |
Here are a couple links to practice chemical equations and nomenclature.
Wednesday, November 11, 2015
Percent Composition, Molecular Formulas, and Empirical Formulas
Today in class, we learned a lot of things. At first, I found the new concepts very easy, but as we continued, they got harder and harder. First, we practiced percent composition. I slightly remembered this from earlier in the school year, and remembered some of the steps as we were doing the questions. Below, I posted some practice questions that I can use while studying for the exam. Next, we learned about Molecular formulas vs. Empirical formulas. Molecular formulas can be Empirical formulas if they can not be reduced any more. For example, the molecular formula of a compound may be P4O10 but the empirical formula would be P2O5. There are more practice problems below for those formulas and other practice quizzes for the questions we practiced with these formulas in class today.
Percent Composition Link
Molecular and Empirical Practice
Calculate empirical formula with percent composition data given
Percent Composition Link
Molecular and Empirical Practice
Calculate empirical formula with percent composition data given
Monday, November 9, 2015
Hydrate Lab
In class today, my partner and I successfully passed the pre-lab quiz for the Hydrate lab. I was really nervous that I wouldn't pass, but was very relieved once Mrs. Frankenberg approved of our answers. After we got our safety goggles, my partner and I picked a station. My partner measured the mass of the test tube and we both recorded it in the data table (picture below). After about 2 cm of CuSO4 hydrate was placed in the test tube, we recorded the mass and heated the blue solution until is turned whiteish-gray, making sure that it did not burn. After that we measured it and heated it again to ensure that we evaporated all the water in the solution. Again, we measured its mass and filled in our data table. Then, Mrs. Frankenberg looked at our data and said it looked good. So, my partner and I cleaned up our lab. However, we made the mistake of cleaning our test tube with the anhydrous with water. It reacted and hardened, making it very hard to clean. Next time, we definitely won't add water and will just throw the ashes in that trash-bin!
Here are a couple pictures of that lab below:
Here are a couple pictures of that lab below:
Thursday, November 5, 2015
Molar Mass
We learned how to calculate the molar mass of a compound in class today. I found the concept easy to learn. However, I do need to review my polyatomic ions.
This is one example we did in class. We still need to round to the correct number of significant figures in the end.
Mrs. Frankenberg also taught us the diatomic ions. They are hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine. When these elements are sitting alone, you must double their mass from the periodic table to find their molar mass.
For example, O2 = 2(16.00) = 32.00 g/mol
Below, I posted a picture of the polyatomic ions that I can use to study and a link to practice calculating the molar mass of compounds before the exam.
molar mass quiz
This is one example we did in class. We still need to round to the correct number of significant figures in the end.
Magnesium chloride = MgCl2 = 1(24.31) + 2(35.45)
= 95.21 g/mol
For example, O2 = 2(16.00) = 32.00 g/mol
Below, I posted a picture of the polyatomic ions that I can use to study and a link to practice calculating the molar mass of compounds before the exam.
 |
| http://crescentok.com/staff/jaskew/isr/tigerchem/naming/a85.htm |
Wednesday, November 4, 2015
Chemical Composition Notes
In class today, we took notes over chemical composition. I learned that a mole is a quantity of measurements and I was reminded that a mole is equivalent to 6.02 x 10^23 representative particles. Mrs. Frankenberg showed us how whole numbers tell us molar quantity in an equation. Here is an example that she showed us in class to demonstrate the concept:
2H2O -----> 2H2 + O2
2 moles 2 moles 1 mole
Next, we practiced problems with moles, and she gave us a handy conversion chart to use while we calculate:

Below are examples we did together in class. Note that we still need to round to the correct number of significant numbers in the end.
Here are a couple websites with more practice problems for converting with moles:
Chemteam: grams to moles
Chemteam: moles to grams
2H2O -----> 2H2 + O2
2 moles 2 moles 1 mole
Next, we practiced problems with moles, and she gave us a handy conversion chart to use while we calculate:

Below are examples we did together in class. Note that we still need to round to the correct number of significant numbers in the end.
Here are a couple websites with more practice problems for converting with moles:
Chemteam: grams to moles
Chemteam: moles to grams
Monday, November 2, 2015
Pretest Reaction
In chemistry class today, we took a pretest over our next unit: Chemical Composition. I thought the test was difficult. However, once I learn the concepts in class, I believe that I will have a better understanding of what's on the exam. A couple terms I remember from the test were empirical formulas and finding the percent by mass. I have a couple pictures and links below that help explain these concepts.
 |
| http://quantummechanics.mchmultimedia.com/2011/general-chemistry-general-chemistry/004-empirical-formula-in-chemistry/ |
 |
| http://quantummechanics.mchmultimedia.com/2011/general-chemistry-general-chemistry/004-empirical-formula-in-chemistry/ |
Tuesday, October 27, 2015
Dimensional Analysis
In class, we took notes over dimensional analysis. Dimensional analysis is when you convert one quantity to another. As we performed the equations, we also had to recognize how many significant numbers were in it in order to apply that number of significant numbers to the overall answer. One rule to remember is that when an exact quantity is in the problem, it is considered infinite, and is not counted as a significant number. An example of an exact quantity is 1 inch= 2.54 centimeters. Below, I posted a couple examples of dimensional analysis that we did in class. In the picture, you can see where we crossed out units of measurement as we worked our way through the problem. Also, there is a link of practice problems below the picture that I will use to study for the exam.
Dimensional Analysis Quiz with Conversion Charts
Dimensional Analysis Quiz with Conversion Charts
Monday, October 26, 2015
Quiz Relfection
Today we took a quiz over our measurement unit in class. I think I did okay. However, there were a few questions I wasn't too sure about, and I began to second guess myself. I especially doubted myself on multiplying, dividing, adding, and subtracting significant numbers. In addition, I had trouble with a question asking about a pure substance. So, I went to find more practice questions online that combine multiplying/dividing and adding/subtracting with significant numbers, and a page explaining more about pure substances, elements, and compounds. I plan on looking back at these links as I study for our upcoming exam.
Chem Team: Elements and Compounds
Practice on Significant Numbers in Operations
Practice Problems on Working with Significant Numbers
Chem Team: Elements and Compounds
Practice on Significant Numbers in Operations
Practice Problems on Working with Significant Numbers
Thursday, October 22, 2015
Mole
Over fall break, I sewed
a stuffed mole in honor of Mole Day. On the paper with the patterns for the
stuffed mole, it briefly explained what a scientific mole was. However, I
decided to research the mole more to fully understand its significance. The Vision Learning website was very helpful. It
explained how a mole is simply a word that represents a measurement. It
compared it to a "dozen" standing for 12. The website furthered
explained that a mole is equivalent to 6.02
x 1023 and stressed its greatness. In addition,
the website described how "A sample of any element with
a mass equal to that element's atomic weight (in grams)
will contain precisely one mole of atoms (6.02 x
1023 atoms). So, if an element had an atomic weight of 6.00 g., that
amount of the element is equivalent to one mole of that element's atoms. I
found this website very interesting and detailed, helping me fully
understand what a mole is through examples and history.
Vision Learning- Mole
 |
| http://www.jabebo.com/Scienceset.htm |
Tuesday, October 20, 2015
Pre-test Reflection
Today in Chemistry class we completed the pre-test for our measurement unit. I did not know the majority of the concepts on the test. However, I think I will be able to perform all problems correctly once I know what certain terms mean and how to convert measurements. Two concepts that kept appearing on the test were significant figures and converting measurements, such as miles to km. Below, I found two measurement conversion charts that I can look back on when I study. One converts only length and the other converts a variety of measurements that will come in handy when I'm converting measurements for my Last Meal Project. In addition, I included a couple websites explaining what significant figures are and how to determine which digits are significant in a measurement. The second website also has practice problems for adding and subtracting significant figures.
Khan Academy Video Explaining Significant Figures
 |
| http://www.math-salamanders.com/measure-conversion-chart.html |
.png) |
| http://www.almanac.com/content/table-measurements |
Tutorial on the Use of Significant Numbers with practice problems
Friday, October 9, 2015
Aspirin Lab
 |
| Buchner Funnel |
 |
| Set- up |
 |
| Scale |
 |
| Crystals the next morning |
 |
| Crystals the next morning |
 |
| Ending Product |
Thursday, October 1, 2015
Unit 2 Test Reflection
Today, our chemistry class completed the atomic structure and radioactivity unit by taking the final unit test. Throughout the past few weeks, I have learned several things about average atomic mass, atomic and mass number, radioactivity, fission and fusion, and the scientists who discovered the structure of an atom. I believe that I was well prepared for the test we took today. There were only a couple questions that I wasn't sure about. One of the questions had to do with half-life. Half-life is the only concept that I am a bit shaky on. So, before we move on to the next unit, I will make sure to practice more half-life questions until I fully comprehend them. Other than that, I think I did well and hope to do just as well in the next unit!
Wednesday, September 30, 2015
Celestial Body Project Reflection
I discovered a lot of new information during the project we just finished. It is absolutely astounding to think how big the universe. As humans, we think Earth is huge. Yet, it is no comparison to our sun, much less the giant stars that burn farther out in the universe. It is also amazing that scientists have the technology that can reach several light-years in the universe to discover all the information we researched. It is all so unimaginable.
While finishing up with my project, I came across a couple videos that helped "click" all the information I found together (links below). In the first video, a man explains the meaning of all the stellar classification letters. While researching, I was able to find the stellar classifications easily. However, I never really knew what all the letter and numbers translated to. After watching the video, I was able to decipher what all the classification meant, which came in handy. Later on, I discovered another video that fully explains fusion within stars and how each star radiates different elements and how atomic nuclei combine and form new elements in the star. I know now how the emission spectrum applies to the project. I feel even more educated on celestial bodies now that I have reinforced my learning with these videos and can see how they apply to chemistry.
Stellar Classification Video Link
Fusion in Stars Link
While finishing up with my project, I came across a couple videos that helped "click" all the information I found together (links below). In the first video, a man explains the meaning of all the stellar classification letters. While researching, I was able to find the stellar classifications easily. However, I never really knew what all the letter and numbers translated to. After watching the video, I was able to decipher what all the classification meant, which came in handy. Later on, I discovered another video that fully explains fusion within stars and how each star radiates different elements and how atomic nuclei combine and form new elements in the star. I know now how the emission spectrum applies to the project. I feel even more educated on celestial bodies now that I have reinforced my learning with these videos and can see how they apply to chemistry.
Stellar Classification Video Link
Fusion in Stars Link
Monday, September 28, 2015
Half-life Class Notes and Forensic Lab
In Chemistry class today, we took notes on half-life and prepared for our Forensic Lab. While taking our notes, I surprisingly remembered several things that I believe I learned in 8th grade. However, I did learn a couple new and helpful things. First, I did not know that the decayed part of a half-life sample becomes stable. I simply thought it just disappeared or became a totally different element. In addition, although it sounds obvious, I didn't realize that you could quickly calculate half-life questions by using exponents in the denominator of the fraction. Previously, I was dreading the thought of dividing a sample multiple times to find the resulting amount of mass in a sample. Now that I know this trick, I will be able to calculate the half-life and amount of samples quickly, decreasing errors.
After completing notes, we cut up a piece of printed paper into 576 pieces for our Forensic Lab. Since we didn't have time to finish the lab in class, I performed it at home. To begin, I took 567 "atoms "(pieces of paper), shook them up, dumped them out, and counted the pieces with their white side up. These pieces represented the number of atoms that had decayed. I then put the "decayed" atoms in a bag and placed the rest of the pieces of paper facing the other way (representing the number of atoms that remained in the sample) back in the cup and repeated these steps 5 more times. Overall, the experiment gave me a visual and physical representation of half-life that reinforced my understanding of the concept. I plan on using the practice problems on the website that I posted a link to in order to practice more half-life questions before my next test. I also included pictures from the Forensic Lab I performed today.
Link to half-life practice questions
Thursday, September 24, 2015
Radioactive Decay Notes
In Chemistry class today, we took notes over radioactive decay. The notes were surprisingly easy to understand. However, I still went to look up more information on the subject and found these helpful pictures from the link I'll put below. The information on the website will also help me later when studying for a test.
Types of Radioactive Decay
Types of Radioactive Decay
 |
| What materials block the different particles |
 |
| Difference between particles |
Wednesday, September 23, 2015
Post-Quiz #1 Reflection
Today, we took our first weekly quiz in our atomic structure and radioactivity unit. I believe that I did very well. However, there was a question that stumped me, involving the different scientists who studied atomic theory. On one question, it showed a picture of an atom and asked which scientist it corresponded with. The atom had a dashed line on the outer edge and contained a nucleus. So, I wasn't sure if it was Rutherford's model or the current atomic model (with the dashed line representing the cloud). Later, I researched the different models, and found that the answer was most likely Rutherford's model.
 |
| JJ Thompson's model https://the-history-of-the-atom.wikispaces.com/J.J.+Thomson?responseToken=0962b7a86afac09cb5b97188b753b553a |
 |
| Current model http://www.particleadventure.org/modern_atom.html |
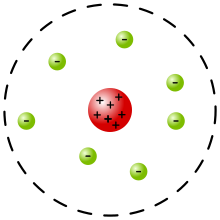 |
| Rutherford's model https://en.wikipedia.org/wiki/Rutherford_model |
I think the cloud model will be more obvious on the next test, and I won't confuse the two models.
Here are a couple links that go into deeper depth talking about each of the scientists' theories. I plan on looking back at these links before our next test.
Tuesday, September 22, 2015
Beanium lab
Today, we performed the Beanium lab in Chemistry class. With a partner, we sorted a bag of different types of beans into groups with the similar looking beans to represent Beanium isotopes.
Next, we counted the number of each type of Beanium isotopes and recorded our data into the table provided to us by Mrs. Frankenberg. We then measured the total mass (g.) of all the atoms of each Beanium isotope with a balance and a small plastic cup. With this information, we were able to calculate the average mass (g.) of each isotope and the percent abundance of each isotope. Finally, we were able find out the overall average atomic mass of Beanium with all of our resulting data. This lab gave me a hands on experience that clearly and visually demonstrated the whole process of calculating the average atomic mass of an atom, allowing me to understand the whole process from a different perspective. Here are some pictures from the lab below:
Next, we counted the number of each type of Beanium isotopes and recorded our data into the table provided to us by Mrs. Frankenberg. We then measured the total mass (g.) of all the atoms of each Beanium isotope with a balance and a small plastic cup. With this information, we were able to calculate the average mass (g.) of each isotope and the percent abundance of each isotope. Finally, we were able find out the overall average atomic mass of Beanium with all of our resulting data. This lab gave me a hands on experience that clearly and visually demonstrated the whole process of calculating the average atomic mass of an atom, allowing me to understand the whole process from a different perspective. Here are some pictures from the lab below:
Thursday, September 17, 2015
How to Calculate the Percent Composition of a Compound
One thing to remember when calculating the percent composition of components in a compound is that: percent composition= proportion by mass
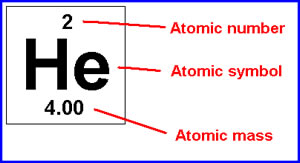
For example, calculate the composition of each component in Potassium sulfide:
First, determine the chemical formula of this compound: K1+ S2- = K2S
Next, calculate the average mass of each component of the compound. The average atomic mass of en element is underneath the symbol on the periodic table. This picture will help you find it:

K2 = 2(39.10)= 78.2
S= 32.07
Now, add up the masses to get the total atomic mass of the compound:
78.2+32.07= 110.27 g/mol
To find the percentage of Potassium in the compound, divide: 78.2/110.27= 70.92%
To find the percentage of Sulfur in the compound, divide: 32.07/110.27= 29.10%
So, Potassium sulfide is 70.92% Potassium and 29.10% Sulfur.
So, Potassium sulfide is 70.92% Potassium and 29.10% Sulfur.
Picture Resource: http://www.differencebetween.info/difference-between-atomic-mass-and-atomic-weight
Tuesday, September 15, 2015
Pre-Test Reflection
Today, in chemistry class, we took the pre-test for the atomic structure and radioactivity unit. I did not understand most of it, but I saw a lot of repeating information and terms that I will need to know in order to pass the quizzes and tests. Throughout the test, I saw a lot of questions about alpha, beta, and gamma radiation, and how the mass number and number of protons and neutrons change when elements undergo these radiations. I also saw many questions asking to calculate half-life. I hope to learn all of this information in the atomic structure and radioactivity unit these upcoming weeks. For now, I will check out these web-pages in order to get a little background information before taking class notes.
Monday, September 14, 2015
Frontier Chemistry Reflection
Overall, I learned a lot from researching my Frontier Chemistry Project and exploring nature to find different plants. I had never realized how many different types of wildflowers and trees were in our area, and how similar several of them were. In addition, I had never realized that most of the flora could be used for a wide range of medicinal uses. Beforehand, I had known that plants were used in early history to cure illnesses, but I never thought more about it. This project caused me to discover information that I would have never found out individually. It's funny that I can now recognize several plants on the side of the road as I'm driving, and I know how I can use them. I feel confident that I could help someone or myself if I were stranded in our area without a phone and needed immediate medical attention.
Naming Binary Compounds
Naming Type One, Two, and Three Binary Compounds Flow-Chart
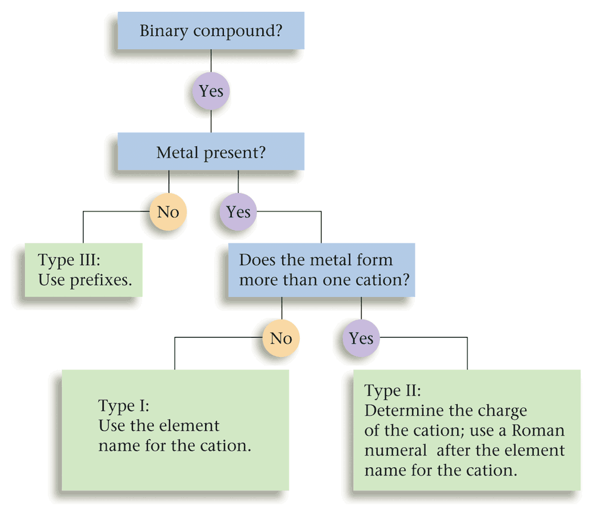 |
| This flow-chart was very useful in helping me identify if a compound was either type one, two, or three. It allowed me to consider all options in order to pick the correct category of binary compound, and summarized all of the considerations that ran through my head when completing a nomenclature question. |
One example that demonstrates how this flow-chart operates is if I was determining if Sodium fluoride was a type one, two or three binary compound. This first question that would come to my head was if it contained a metal. It does contain a metal, since Sodium is an alkali metal. If it did not contain a metal, it would be a type three binary compound, Next, I would ask myself if the metal had a definite charge. Since Sodium's charge is always 1+, it does have a definite charge, and it is, therefore, a type 1 binary compound. If the cation did not have a definite charge, it would have been a type three binary compound.
Thursday, August 20, 2015
Introduction Page
Welcome to my Frontier Chemistry Blog! I am Lilly Apperson and a junior attending high school. I am very involved in school. I am a swimmer on my girls' high school swim team and a member of STUCO, HOSA, and NHS. After high school, I hope to study medicine. I love the beach and going shopping. I hope you enjoy my chemistry blog and find my information interesting and helpful!

Subscribe to:
Comments (Atom)













