-Formation of a solid
-Formation of water
-Formation a gas
-Transfer of electrons
There is a link to a video below that explains these and indications of chemical reactions
However, we mainly focused on the formation of a solid today, which is also known as precipitate. With these, double replacement precipitation reactions take place. In a double replacement reaction, 2 compounds replace 2 compounds by having the positive ions (cations) switch with each other. The format of this reaction looks like this:
 |
| http://socratic.org/questions/is-this-reaction-a-double-replacement-reaction-fe-c5h5-2-bf4-nab-c6f5-4-fe-c5h5- |
In addition, for this type of reaction to take place, the compounds that are the reactants must be ionic and aqueous and one of the products must be a solid. We must memorize the solubility rules to check if a chemical equation has a driving force.
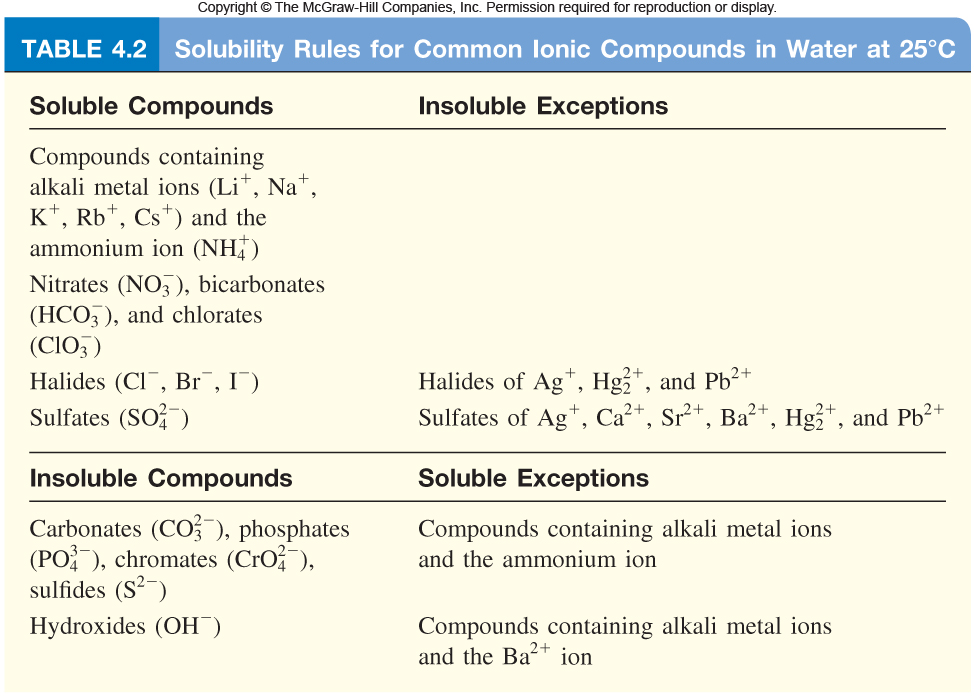 |
| http://highered.mheducation.com/olcweb/cgi/pluginpop.cgi?it=jpg::::::/sites/dl/free/0023654666/650262/Solubility_Rules_4_02.jpg::Solubility%20rules |
Lily your post clarified any questions I had with double replacement reactions, driving forces, and solubility rules. The video you have on driving forces was very helpful.
ReplyDeleteI like this weblog so much, saved to my bookmarks . driving classes
ReplyDelete