In class yesterday, we learned about redox reactions and the different types. In redox reactions, electrons are transferred from the metal to the nonmetal. If a species loses electrons, it is said to be oxidized, and it is the reducing agent. On the other hand, if a species gains electrons,it is said to be reduced, and it is the oxidizing agent. A way to remember this is:
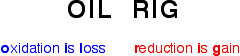 |
| http://www.chemguide.co.uk/inorganic/redox/definitions.html |
In redox-single replacement reactions, the metals change particles and the driving force is the transfer of electrons. The reaction is based on a reactivity series. Something to remember is "like attacks like." So, a metal attacks a metal, and a nonmetal attacks nonmetals. Below is an example:
 |
| http://makahiki.kcc.hawaii.edu/chem/single_displ_rxn.html |
Next, we practiced synthesis reactions. Synthesis reactions occur when two or more reactants combine to form one product. The general formula is A + B = AB. Decomposition reactions are the opposite, so one reactant produces two or more products. The general formula for decomposition reactions is AB = A + B.
Lastly, we reviewed combustion reactions as our last redox reaction. In a combustion reaction, the reactants always include water, and produce carbon dioxide and water. Below is an example:
 |
| http://www.angelfire.com/un/sch3u1/combustion.html |
Here are a couple videos that further explain redox reactions:
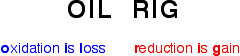

Lilly, I love the mnemonic device and images you included in this! I will use them to study for the final for sure. I love your blog.
ReplyDeleteThis comment has been removed by the author.
ReplyDeleteGreat post Lilly! I found the pictures you included in this post to be very beneficial in understanding oxidation, reduction, and combustion! I also found the videos you linked to be helpful as well.
ReplyDeleteThanks for such a great post Lilly! your explanations are very clear and concise and I found it very helpful to understanding these different types of reactions. Also, a picture of each explanation is not something I see very often, yet is something that is very beneficial to me. Your links were also very helpful in going into more depth when I did not understand certain details.
ReplyDeleteThank you for posting this as it was very detailed and specific as well as informative. The pictures especially give you a clear idea of the reactions.
ReplyDelete