For example, calculate the composition of each component in Potassium sulfide:
First, determine the chemical formula of this compound: K1+ S2- = K2S
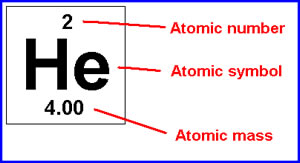
Next, calculate the average mass of each component of the compound. The average atomic mass of en element is underneath the symbol on the periodic table. This picture will help you find it:
K2 = 2(39.10)= 78.2
S= 32.07
Now, add up the masses to get the total atomic mass of the compound:
78.2+32.07= 110.27 g/mol
To find the percentage of Potassium in the compound, divide: 78.2/110.27= 70.92%
To find the percentage of Sulfur in the compound, divide: 32.07/110.27= 29.10%
So, Potassium sulfide is 70.92% Potassium and 29.10% Sulfur.
So, Potassium sulfide is 70.92% Potassium and 29.10% Sulfur.
Picture Resource: http://www.differencebetween.info/difference-between-atomic-mass-and-atomic-weight
Lilly, I found this post very educational, as I have never heard of calculating the percent composition of a compound. After reading your post, I am looking forward to learning more about this topic as the class progresses. I really appreciate the insight you provided about this topic by including an actual example of how to calculate it. This allowed me to better understand it, and throughout the unit, I can come back to this post if I am ever confused about the topic. The only thing I would suggest to add to this post would maybe be to explain what the "average atomic mass" of an element actually means. I am just a little unclear on what exactly this term refers to, and other readers could have the same issue. Overall, I found this post really helpful and thank you for sharing.
ReplyDeleteI found your post very helpful as it went in depth about the composition of compounds. The diagrams and your explanations allowed me to better understand this topic, as before I did not fully understand. I think this post was very educational and helpful in every aspect, thank you for sharing!
ReplyDelete