I discovered a lot of new information during the project we just finished. It is absolutely astounding to think how big the universe. As humans, we think Earth is huge. Yet, it is no comparison to our sun, much less the giant stars that burn farther out in the universe. It is also amazing that scientists have the technology that can reach several light-years in the universe to discover all the information we researched. It is all so unimaginable.
While finishing up with my project, I came across a couple videos that helped "click" all the information I found together (links below). In the first video, a man explains the meaning of all the stellar classification letters. While researching, I was able to find the stellar classifications easily. However, I never really knew what all the letter and numbers translated to. After watching the video, I was able to decipher what all the classification meant, which came in handy. Later on, I discovered another video that fully explains fusion within stars and how each star radiates different elements and how atomic nuclei combine and form new elements in the star. I know now how the emission spectrum applies to the project. I feel even more educated on celestial bodies now that I have reinforced my learning with these videos and can see how they apply to chemistry.
Stellar Classification Video Link
Fusion in Stars Link
Wednesday, September 30, 2015
Monday, September 28, 2015
Half-life Class Notes and Forensic Lab
In Chemistry class today, we took notes on half-life and prepared for our Forensic Lab. While taking our notes, I surprisingly remembered several things that I believe I learned in 8th grade. However, I did learn a couple new and helpful things. First, I did not know that the decayed part of a half-life sample becomes stable. I simply thought it just disappeared or became a totally different element. In addition, although it sounds obvious, I didn't realize that you could quickly calculate half-life questions by using exponents in the denominator of the fraction. Previously, I was dreading the thought of dividing a sample multiple times to find the resulting amount of mass in a sample. Now that I know this trick, I will be able to calculate the half-life and amount of samples quickly, decreasing errors.
After completing notes, we cut up a piece of printed paper into 576 pieces for our Forensic Lab. Since we didn't have time to finish the lab in class, I performed it at home. To begin, I took 567 "atoms "(pieces of paper), shook them up, dumped them out, and counted the pieces with their white side up. These pieces represented the number of atoms that had decayed. I then put the "decayed" atoms in a bag and placed the rest of the pieces of paper facing the other way (representing the number of atoms that remained in the sample) back in the cup and repeated these steps 5 more times. Overall, the experiment gave me a visual and physical representation of half-life that reinforced my understanding of the concept. I plan on using the practice problems on the website that I posted a link to in order to practice more half-life questions before my next test. I also included pictures from the Forensic Lab I performed today.
Link to half-life practice questions
Thursday, September 24, 2015
Radioactive Decay Notes
In Chemistry class today, we took notes over radioactive decay. The notes were surprisingly easy to understand. However, I still went to look up more information on the subject and found these helpful pictures from the link I'll put below. The information on the website will also help me later when studying for a test.
Types of Radioactive Decay
Types of Radioactive Decay
 |
| What materials block the different particles |
 |
| Difference between particles |
Wednesday, September 23, 2015
Post-Quiz #1 Reflection
Today, we took our first weekly quiz in our atomic structure and radioactivity unit. I believe that I did very well. However, there was a question that stumped me, involving the different scientists who studied atomic theory. On one question, it showed a picture of an atom and asked which scientist it corresponded with. The atom had a dashed line on the outer edge and contained a nucleus. So, I wasn't sure if it was Rutherford's model or the current atomic model (with the dashed line representing the cloud). Later, I researched the different models, and found that the answer was most likely Rutherford's model.
 |
| JJ Thompson's model https://the-history-of-the-atom.wikispaces.com/J.J.+Thomson?responseToken=0962b7a86afac09cb5b97188b753b553a |
 |
| Current model http://www.particleadventure.org/modern_atom.html |
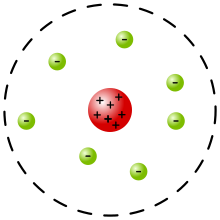 |
| Rutherford's model https://en.wikipedia.org/wiki/Rutherford_model |
I think the cloud model will be more obvious on the next test, and I won't confuse the two models.
Here are a couple links that go into deeper depth talking about each of the scientists' theories. I plan on looking back at these links before our next test.
Tuesday, September 22, 2015
Beanium lab
Today, we performed the Beanium lab in Chemistry class. With a partner, we sorted a bag of different types of beans into groups with the similar looking beans to represent Beanium isotopes.
Next, we counted the number of each type of Beanium isotopes and recorded our data into the table provided to us by Mrs. Frankenberg. We then measured the total mass (g.) of all the atoms of each Beanium isotope with a balance and a small plastic cup. With this information, we were able to calculate the average mass (g.) of each isotope and the percent abundance of each isotope. Finally, we were able find out the overall average atomic mass of Beanium with all of our resulting data. This lab gave me a hands on experience that clearly and visually demonstrated the whole process of calculating the average atomic mass of an atom, allowing me to understand the whole process from a different perspective. Here are some pictures from the lab below:
Next, we counted the number of each type of Beanium isotopes and recorded our data into the table provided to us by Mrs. Frankenberg. We then measured the total mass (g.) of all the atoms of each Beanium isotope with a balance and a small plastic cup. With this information, we were able to calculate the average mass (g.) of each isotope and the percent abundance of each isotope. Finally, we were able find out the overall average atomic mass of Beanium with all of our resulting data. This lab gave me a hands on experience that clearly and visually demonstrated the whole process of calculating the average atomic mass of an atom, allowing me to understand the whole process from a different perspective. Here are some pictures from the lab below:
Thursday, September 17, 2015
How to Calculate the Percent Composition of a Compound
One thing to remember when calculating the percent composition of components in a compound is that: percent composition= proportion by mass
For example, calculate the composition of each component in Potassium sulfide:
First, determine the chemical formula of this compound: K1+ S2- = K2S
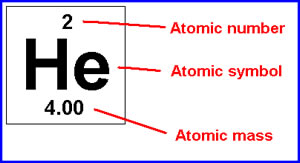
Next, calculate the average mass of each component of the compound. The average atomic mass of en element is underneath the symbol on the periodic table. This picture will help you find it:

K2 = 2(39.10)= 78.2
S= 32.07
Now, add up the masses to get the total atomic mass of the compound:
78.2+32.07= 110.27 g/mol
To find the percentage of Potassium in the compound, divide: 78.2/110.27= 70.92%
To find the percentage of Sulfur in the compound, divide: 32.07/110.27= 29.10%
So, Potassium sulfide is 70.92% Potassium and 29.10% Sulfur.
So, Potassium sulfide is 70.92% Potassium and 29.10% Sulfur.
Picture Resource: http://www.differencebetween.info/difference-between-atomic-mass-and-atomic-weight
Tuesday, September 15, 2015
Pre-Test Reflection
Today, in chemistry class, we took the pre-test for the atomic structure and radioactivity unit. I did not understand most of it, but I saw a lot of repeating information and terms that I will need to know in order to pass the quizzes and tests. Throughout the test, I saw a lot of questions about alpha, beta, and gamma radiation, and how the mass number and number of protons and neutrons change when elements undergo these radiations. I also saw many questions asking to calculate half-life. I hope to learn all of this information in the atomic structure and radioactivity unit these upcoming weeks. For now, I will check out these web-pages in order to get a little background information before taking class notes.
Monday, September 14, 2015
Frontier Chemistry Reflection
Overall, I learned a lot from researching my Frontier Chemistry Project and exploring nature to find different plants. I had never realized how many different types of wildflowers and trees were in our area, and how similar several of them were. In addition, I had never realized that most of the flora could be used for a wide range of medicinal uses. Beforehand, I had known that plants were used in early history to cure illnesses, but I never thought more about it. This project caused me to discover information that I would have never found out individually. It's funny that I can now recognize several plants on the side of the road as I'm driving, and I know how I can use them. I feel confident that I could help someone or myself if I were stranded in our area without a phone and needed immediate medical attention.
Naming Binary Compounds
Naming Type One, Two, and Three Binary Compounds Flow-Chart
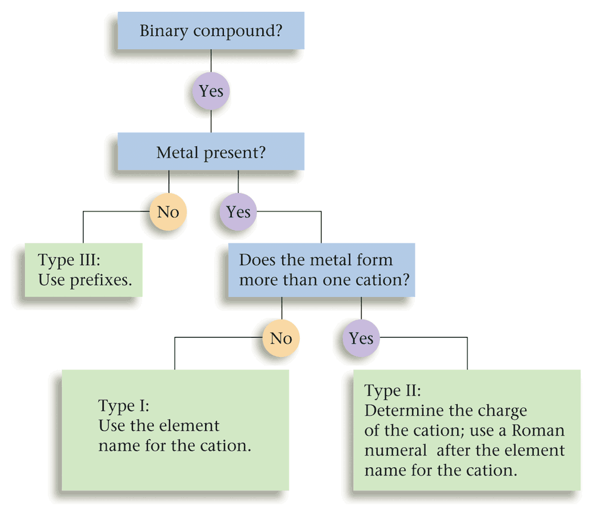 |
| This flow-chart was very useful in helping me identify if a compound was either type one, two, or three. It allowed me to consider all options in order to pick the correct category of binary compound, and summarized all of the considerations that ran through my head when completing a nomenclature question. |
One example that demonstrates how this flow-chart operates is if I was determining if Sodium fluoride was a type one, two or three binary compound. This first question that would come to my head was if it contained a metal. It does contain a metal, since Sodium is an alkali metal. If it did not contain a metal, it would be a type three binary compound, Next, I would ask myself if the metal had a definite charge. Since Sodium's charge is always 1+, it does have a definite charge, and it is, therefore, a type 1 binary compound. If the cation did not have a definite charge, it would have been a type three binary compound.
Subscribe to:
Comments (Atom)




