On Wednesday, we received our Stoichiomety quiz grades back and I got a 100%. I was so relieved. My hard studying the night before paid off. Now, I am preparing for the unit exam. Below are a couple helpful links with practice quizzes and tests that will help me learn more about the subject. Hope you find these helpful!
Somerset Academy Practice Test
Glencoe Practice Quiz
Sunday, December 13, 2015
Day 1 and 2 of Copper (II) Chloride and Iron Lab
On Thursday and Friday, my lab partner and I performed an experiment where we produced copper from an iron nail and copper(II) chloride. On day one, after we passed our pre-lab quiz, measured about 4 grams of copper(II) chloride, and placed it in a baby food jar. It was a vibrant blue. Next, we added 50 mL of distilled water to the jar and stirred it until it dissolved. We then placed an iron nail that had been polished with steel wool in the jar to sit overnight. Within in minutes it looked like this...

 You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
In order to separate the copper from the rest of the solution, we pulled out the rest of the iron nail, washing off the remaining copper on the nail with distilled water back into the baby food jar. Then, we siphoned off the solution of iron (II) chloride into the sink, careful not to lose any of the copper the reaction produced. We then added hydrochloric acid to the baby food jar to wash the copper and also siphoned it off into the sink. Lastly, we washed the copper again with distilled water and siphoned off the water. Finally, we left the copper in the baby food jar and the nail on a paper towel to dry. We will finish measurements this week.

 You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
You can see that the copper immediately began to form. On Friday, we came back to the lab and found our baby food jar like this...
In order to separate the copper from the rest of the solution, we pulled out the rest of the iron nail, washing off the remaining copper on the nail with distilled water back into the baby food jar. Then, we siphoned off the solution of iron (II) chloride into the sink, careful not to lose any of the copper the reaction produced. We then added hydrochloric acid to the baby food jar to wash the copper and also siphoned it off into the sink. Lastly, we washed the copper again with distilled water and siphoned off the water. Finally, we left the copper in the baby food jar and the nail on a paper towel to dry. We will finish measurements this week.
Wednesday, December 9, 2015
Limiting Reagent and Excess Reagant
On Monday, we learned how to find the limiting reagent and excess reagent in a chemical reaction. There are two approaches. I prefer to use approach two. Below are the steps:
When solving for the limiting reagent, you must convert both of the reactants in a chemical equation to one of the products, using moles. Whichever conversion results in a smaller product, that is the limiting reagent. It is the limiting reagent because once that reactant runs out, the reaction will end and the other reactant will be left in excess. After determining the limiting reagent, you know the other reactant is the excess reagent. You can figure out how much was left in excess by converting the amount of the product that resulted from the previous limiting reagent conversion back to the amount of the excess reagent. Once you discover how much reacted, you can then subtract that number from the original amount and you will know how much is left after the reaction ends.
This picture show the reactants before and after the reaction, distinguishing which is the limiting reagent and what is leftover and alone as the excess reagent.
 |
| http://www.mhhe.com/physsci/chemistry/chang7/ssg/chap03_9sg.html |
Below is a link that explains limiting and excess reagents and another link with practice problems.
Tuesday, December 8, 2015
New Unit
On Friday, we began our new unit: Stoichiometry. Stoichiometry involves the relationship between the reactants and products in chemical equations. In the lecture, we balanced equations and followed a flow chart (the one below) to find the grams and moles of its different products and reactants.
Here is a video that explains Stoichiometry and its significance and a link to where you can practice simple Stoichiometry problems.
Video
Practice
Here is a video that explains Stoichiometry and its significance and a link to where you can practice simple Stoichiometry problems.
Video
Practice
Monday, November 30, 2015
Classifications
There are three umbrellas of chemical reactions:
- Formation of a solid
- Formation of water
- Transfer of elections
In the formation of a solid, double replacement reactions take place. On the reactants side, both reactants must be aqueous. On the product side, one product is insoluble and one product is soluble.
In the formation of water, a strong acid and a strong base must react in order to form water and a salt. If the acid and base are not strong, they will not split. Double replacement reactions also take place in this type of reaction.
If the reaction does not fit into either of these categories, it is a redox reaction. There are four types of redox reactions: single replacement, synthesis, decomposition, and combustion.
Here is a link to practice recognizing reactions: Classification Quiz
Wednesday, November 25, 2015
Transfer of Electrons: Redox notes
In class yesterday, we learned about redox reactions and the different types. In redox reactions, electrons are transferred from the metal to the nonmetal. If a species loses electrons, it is said to be oxidized, and it is the reducing agent. On the other hand, if a species gains electrons,it is said to be reduced, and it is the oxidizing agent. A way to remember this is:
In redox-single replacement reactions, the metals change particles and the driving force is the transfer of electrons. The reaction is based on a reactivity series. Something to remember is "like attacks like." So, a metal attacks a metal, and a nonmetal attacks nonmetals. Below is an example:
Next, we practiced synthesis reactions. Synthesis reactions occur when two or more reactants combine to form one product. The general formula is A + B = AB. Decomposition reactions are the opposite, so one reactant produces two or more products. The general formula for decomposition reactions is AB = A + B.
Here are a couple videos that further explain redox reactions:
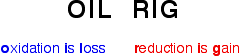 |
| http://www.chemguide.co.uk/inorganic/redox/definitions.html |
 |
| http://makahiki.kcc.hawaii.edu/chem/single_displ_rxn.html |
Lastly, we reviewed combustion reactions as our last redox reaction. In a combustion reaction, the reactants always include water, and produce carbon dioxide and water. Below is an example:
| http://www.angelfire.com/un/sch3u1/combustion.html |
Monday, November 23, 2015
Reactions that form water notes
Today in chemistry class, we took notes over reactions that form water. These reactions include an acid and a base, and the driving force is the production of water. A salt is also produced. For example:
| http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/acidbase.html |
Acids can be strong or weak and bases can be strong or weak.
Strong acids:
- produce H+
- protonate completely
- are strongest when oxygens outnumber the hydrogens by 2 or more
- HCl, HBr, HI
Strong bases:
- contain an -OH anion
- completely disassociate
- All group 1 and 2 metals cations plus an -OH anion are strongest
Weak acids and weak bases:
- do not protonate completely
- are not on the memorized list
Here is a link that further explains strong/weak acids and bases: ChemTeam
Here is a link to practice determining is an acid is strong or weak: about chemistry
Below is also a molecular, complete, and net force equation using strong acids and bases that we solved in class:
Subscribe to:
Posts (Atom)




