 |
| http://agaul01.blogspot.com/2014/04/boyles-charles-law-in-relation-to.html |
Here is his formula too. It is easy to plug in the information you get from a problem, but you have to make sure that you convert Celsius to Kelvin if the problem gives you a temperature in Celsius. :
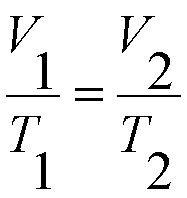 |
| https://www.clippard.com/cms/wiki/charless-law |
Below are a couple links that explain Charles' law more and provide some practice math questions:
Chemteam
Practice Problems
Lily I liked this post because your summary of the law was very descriptive but still short. I thought your picture you added was a great addition, it really emphasized the concept of the law.
ReplyDelete