Today in Chemistry, we learned our first Gas Laws Lesson. We focused on Boyle's Law, which only manipulates volume and pressure. It is an inverse relationship, and holds true at a constant temperature. Below is the formula:
 |
| http://www.physbot.co.uk/gas-laws.html |
To represent its inverse relationship, take the picture below into account:
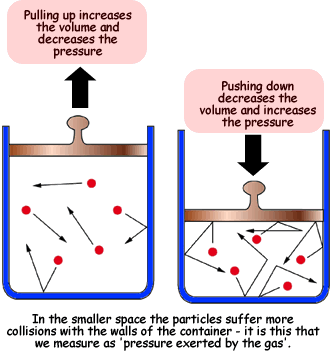 |
| http://www.cyberphysics.co.uk/topics/kinetic_theory/boyle.htm |
As you can see, as the volume decreases, the pressure increases, representing its inverse relationship.
Here are some more links that elaborate on Boyle's Gas Rule:


No comments:
Post a Comment