In our last lesson of the unit, we learned about periodic trends. There are 4 trends that mainly focus on the S and P blocks on the periodic table. First, we learned about atomic size. As you move across a period from left to right, atomic size decreases because there is an increase in protons. Since the protons increasingly attract the electrons, the electrons pull toward the protons more and more, making the size smaller as you go to the right. Here is a chart that displays this trend:
 |
| https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/nonmetallic-elements-21/properties-of-nonmetals-147/atomic-size-569-7507/ |
Next, we discussed ionization energy. Ionization energy is the energy needed to remove an electron from a gaseous state. As you move up and to the right of the periodic table, the ionization energy increases. Here is a chart that displays this concept;
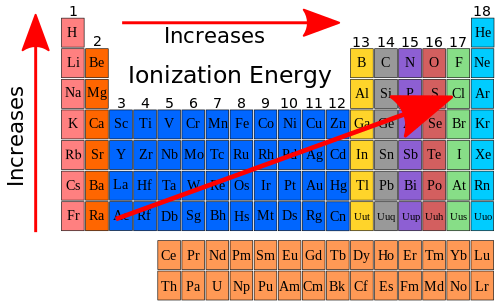 |
| https://en.wikibooks.org/wiki/High_School_Chemistry/Ionization_Energy |
Then, we learned about trends in electron affinity, which is how easy it is to add another electron to an atom. Electron affinity also increases as you move up and to the right of the periodic table. Here is a chart the exemplifies the trend:
 |
| https://en.wikibooks.org/wiki/High_School_Chemistry/Electron_Affinity |
Lastly, we discussed trends in electronegativity, which is the tendency of an atom to draw electrons toward itself when chemically combined with another element. Elements with larger electronegativity tend to pull electrons to themselves when bonded to other elements. It increases as you go up and to the right of the periodic table too. Here is a chart that displays this trend:
 |
| http://chemteacher.chemeddl.org/services/chemteacher/index.php?option=com_content&view=article&id=91 |
Here are a couple links that further explain some of these trends:




This post was very helpful, thank you. I really liked all the pictures they helped a lot for understanding periodic trends. And your explanations were very helpful to understanding them.
ReplyDeleteThank you, this was a really detailed post over the trends with lots of visual aids, and I like the brief summary between each one.
ReplyDeleteThanks for the great post Lilly. I enjoyed how you walked through what defined each trend and where it was at its greatest, as this was something that had to be memorized for the test. I also found it very helpful how you provided a picture for each trend. By separating it, it was much easier to understand and remember them. Thanks again!
ReplyDeleteThis post was great, the amount of pictures included were super good because they were all related and you had great explanations for each of them. I agree with Holly that it was helpful how you separated each trend that way you could understand it better.
ReplyDeleteThe amount and variety of pictures were super helpful to understanding the topic, and your explanation was very thorough and understandable. Great post!
ReplyDeleteLily, I really liked how you included a summary of all the periodic trends in one blogs post. I concluded that electronegativty as well as ionization all increase up and towards the right. After taking the unit exam, I really see how your blog can be of aid to studying for an exam. Keep up the good work.
ReplyDelete