 |
| http://edtech2.boisestate.edu/lindabennett1/502/Compounds%20and%20Naming/Lewis%20Dot.html |
The octet rule technically determines how many electrons are to be placed in the valence shell. An atom can not have more that 8 electrons total in their outer shell. However, there are exceptions. Hydrogen and Helium only have up to two valence electrons, boron requires 6 electrons to be stable, and beryllium only needs 4 electron to be stable.
Another concept we learned today was how to solve electron dot formulas of molecules with the HAVE NEED SHARE method. Below is an example:
Here are a couple websites to further explain and practice these new concepts:
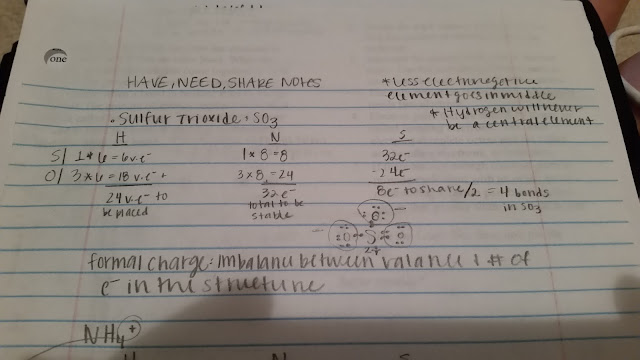
No comments:
Post a Comment