In our last lesson over the unit, we learned about polarity and different types of bonds. With polarity, electrons are not always shared equally between two atoms. It is often the case where one atom may exert more force on the electron cloud than the other. The polarity of an atom is determined by the difference in electronegativity between the atoms. A coavlent bond is nonpolar if the difference between the two is only a 0.2-0.5 difference. A covalent bond in polar if the difference is ~0.5-1.6. All other bonds with a difference higher than 1.6 are most likely ionic. Covalent bonds regularly occur between two nonmetals and sometimes a nonmetal and a meatalloid. An ionic bond in normally between a metal and a nonmetal. Here is an image that displays this concept.
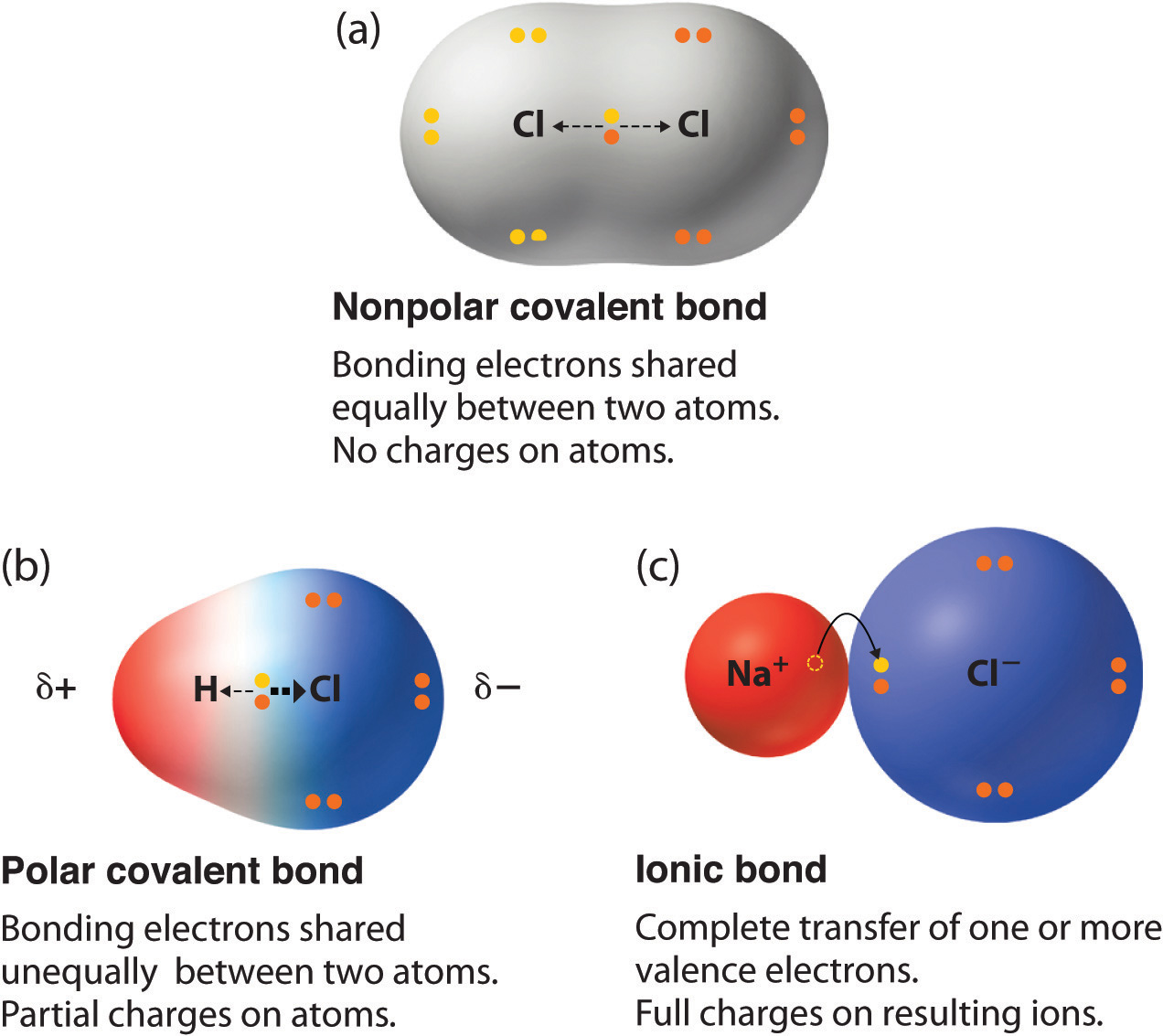 |
| http://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/s12-09-polar-covalent-bonds.html |
Also, here are some links that further explain the topic:


I never quite understood the bonding compatibles between molecules and electrons. From your post I realized that based on the ions present in the elements deciefers the end polarity of the molecule. Also the element that is covalent is a weak bond between electrons while an ionic bond is the complete transfer of electrons.
ReplyDelete