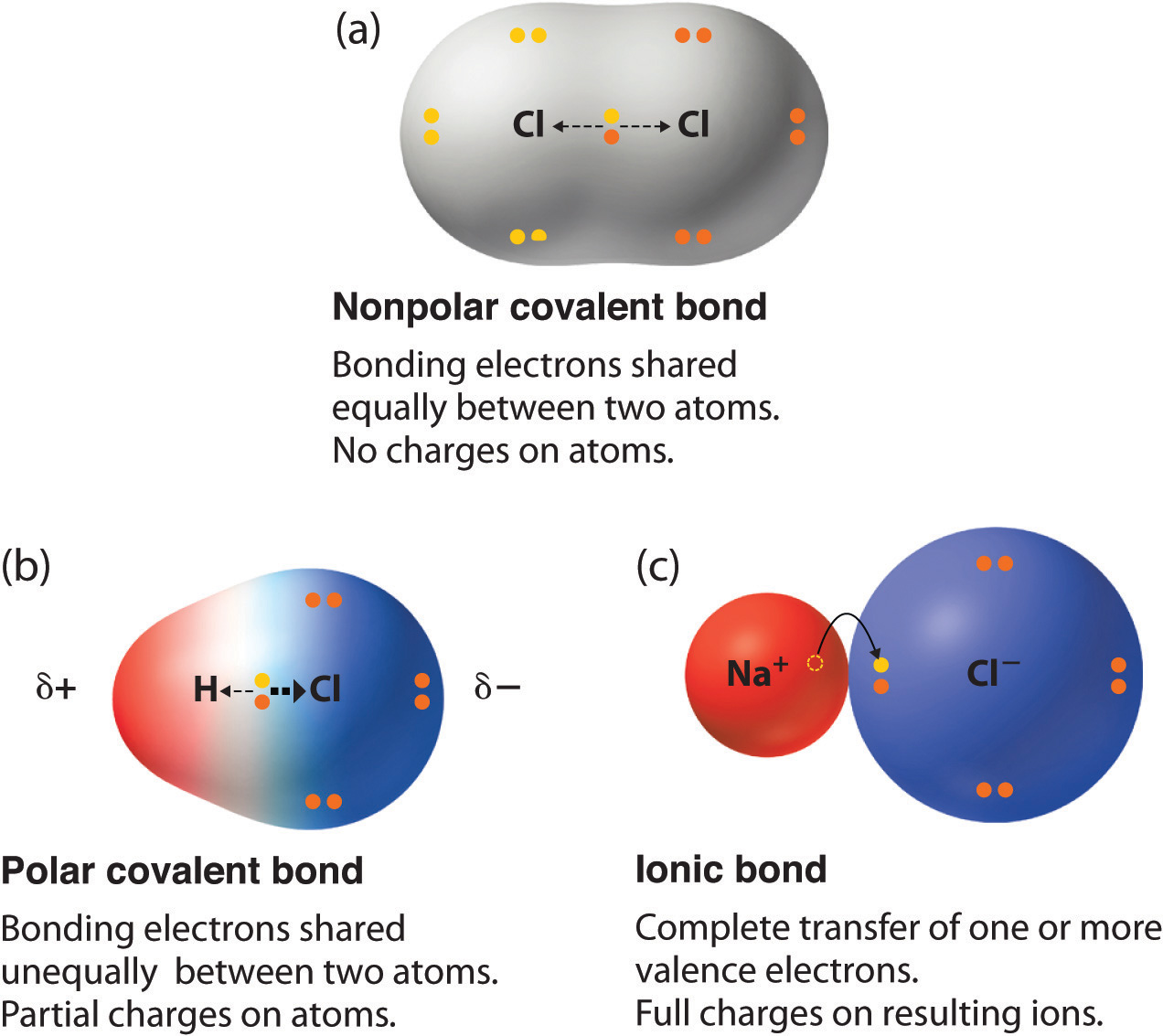
In our last lesson of the unit, we learned about periodic trends. There are 4 trends that mainly focus on the S and P blocks on the periodic table. First, we learned about atomic size. As you move across a period from left to right, atomic size decreases because there is an increase in protons. Since the protons increasingly attract the electrons, the electrons pull toward the protons more and more, making the size smaller as you go to the right. Here is a chart that displays this trend:
 |
| https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/nonmetallic-elements-21/properties-of-nonmetals-147/atomic-size-569-7507/ |
Next, we discussed ionization energy. Ionization energy is the energy needed to remove an electron from a gaseous state. As you move up and to the right of the periodic table, the ionization energy increases. Here is a chart that displays this concept;
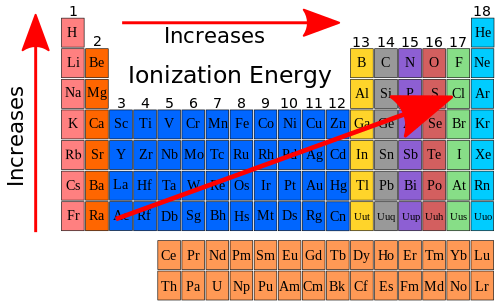 |
| https://en.wikibooks.org/wiki/High_School_Chemistry/Ionization_Energy |
Then, we learned about trends in electron affinity, which is how easy it is to add another electron to an atom. Electron affinity also increases as you move up and to the right of the periodic table. Here is a chart the exemplifies the trend:
 |
| https://en.wikibooks.org/wiki/High_School_Chemistry/Electron_Affinity |
Lastly, we discussed trends in electronegativity, which is the tendency of an atom to draw electrons toward itself when chemically combined with another element. Elements with larger electronegativity tend to pull electrons to themselves when bonded to other elements. It increases as you go up and to the right of the periodic table too. Here is a chart that displays this trend:
 |
| http://chemteacher.chemeddl.org/services/chemteacher/index.php?option=com_content&view=article&id=91 |
Here are a couple links that further explain some of these trends: