To complete all of the questions from this lesson, we used the flow chart below.
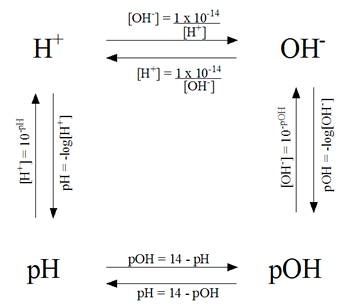 |
| http://www.sciencegeek.net/Chemistry/taters/Unit8pH.htm |
In the chart, the OH- is the hydroxide ions and the H+ is the hydronium ions in a solution. By knowing one of the components on the chart, you can solve the pH, pOH, or the ions of the acids and bases in that particular solution.
An example of a problem that would utilize this chart looks like this. The question regularly involves finding the equilibrium:
 |
| http://pinl.net/vs/ice-tables-for-equilibrium.xhtml |
In this problem, the I stands for INITIAL, which is the original information you are given. The C stand for CHANGE, which is +x, -x, -x most of the time across. Lastly the E is for EQUILIBRIUM. Once you place all of the components in the chart, you add them down. Next, you take those components and put them in the expression on the right, placing the products over the reactants in the solution (not including water) and equal that to the constant. Then, you distrubute the the costant to the fraction to come up with a quadratic formula. Next, with the formula, you place the parts of the formula to form a quadratic equation to find x. After you solve for x, you have the ion concentrations of either the acid or base you were looking for. Finally, if you wanted to find the pH or the pOH of the solution, you place the concentration in the antilog.
Here is a link to practice these types of questions and further explanations:
Explanation
Practice






