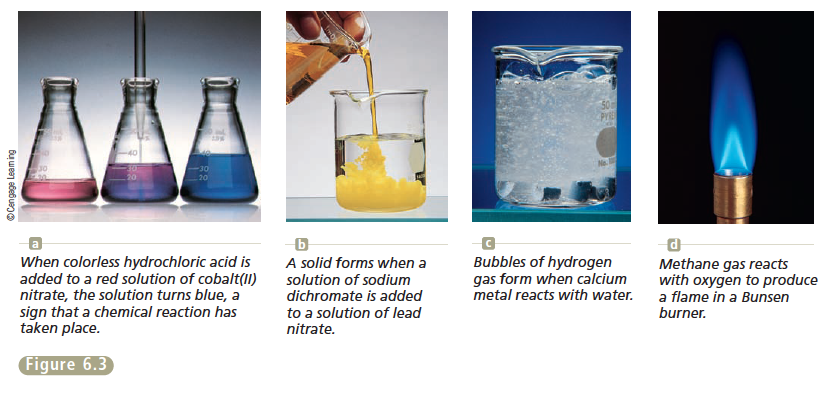
- Formation of a solid
- Formation of water
- Transfer of elections
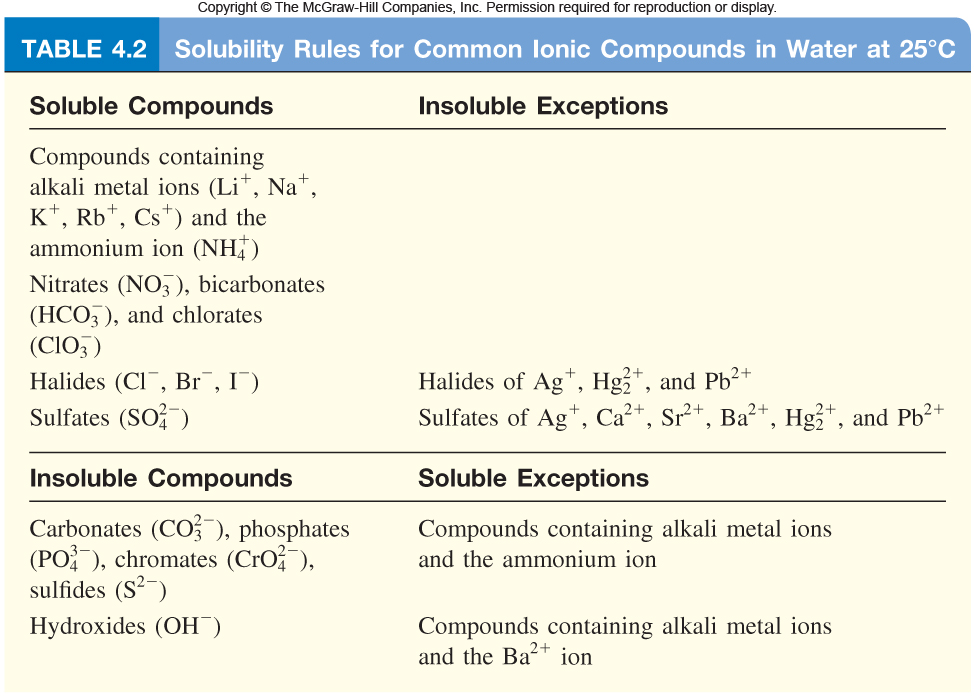
In the formation of a solid, double replacement reactions take place. On the reactants side, both reactants must be aqueous. On the product side, one product is insoluble and one product is soluble.
In the formation of water, a strong acid and a strong base must react in order to form water and a salt. If the acid and base are not strong, they will not split. Double replacement reactions also take place in this type of reaction.
If the reaction does not fit into either of these categories, it is a redox reaction. There are four types of redox reactions: single replacement, synthesis, decomposition, and combustion.
Here is a link to practice recognizing reactions: Classification Quiz